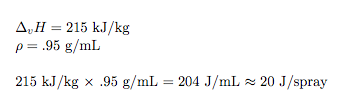No, not that kind of [fun](http://en.wikipedia.org/wiki/Inhalant) – I’m talking about fun with endothermic reactions!
You see – Dust-Off isn’t really compressed air (pressurized Nitrogen is pretty boring) but is in fact liquid [difluoroethane](http://en.wikipedia.org/wiki/Difluoroethane). So what happens when you release a liquid with a boiling point well below that of room temperature (-24.9 °C)?
Lets find out:
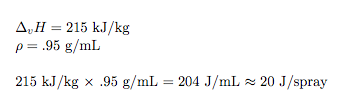
You get a reaction that will absorb an awful lot of energy from it’s surroundings (you can freeze stuff).
Getting more to the point- around here, in the summer, I come across a fair bunch of house spiders and, while I don’t mind them outdoors, can’t have them wandering around indoors. The problem is that they can be pretty tough to catch (especially the small ones) and mashing them makes a mess (especially the big ones). However, with the help of the aforementioned fluorocarbon, dealing with insects is a breeze.
All you need to do is give the can a good shake, tilt sideways and aim and fire. If you do things correctly the nozzle (moistened by shaking) will produce a directed spray of very fine difluoroethane droplets that, due to the their large surface area, vaporize rapidly – making the immediate surroundings very cool.
Bonus: on a humid day, your very likely to see frost form from the spray!